A photocell is a device for generating an electrical current from light (see Figure 1).
Figure 1
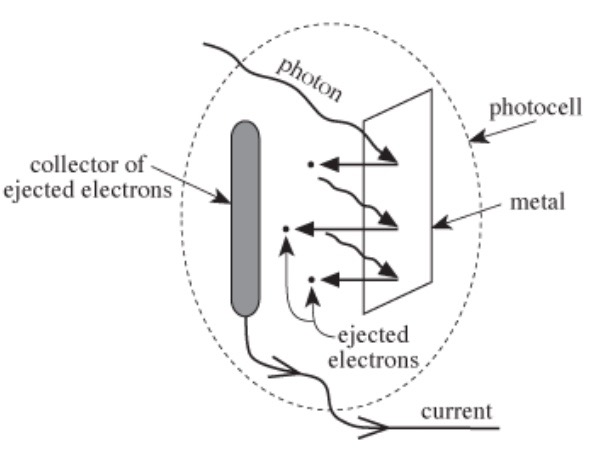
Each photocell contains a metal. A photon of light that strikes the metal can eject an electron from the metal if the photon’s energy exceeds the metal’s work function. The maximum kinetic energy the ejected electron can have is the photon’s energy minus the metal’s work function. The amount of electrical current varies with light’s relative intensity (a measure of the number of photons with a given energy striking the metal each second).
Table 1 shows the results of 9 trials in which a photocell was exposed to light.
Table 1
| Trial |
Energy per photon (eV)* |
Relative intensity of light |
Electrical current (mA)† |
kinetic energy of electron if ejected from metal (eV) |
| 1 |
2.0 |
low |
0 |
0.0 |
| 2 |
2.0 |
medium |
0 |
0.0 |
| 3 |
2.0 |
high |
0 |
0.0 |
| 4 |
4.0 |
low |
29 |
0.9 |
| 5 |
4.0 |
medium |
43 |
0.9 |
| 6 |
4.0 |
high |
60 |
0.9 |
| 7 |
6.0 |
low |
27 |
2.9 |
| 8 |
6.0 |
medium |
40 |
2.9 |
| 9 |
6.0 |
high |
55 |
2.9 |
| *eV = electron volts |
| †mA = milliamps |
When 8.0 eV photons were shone on the photocell, electrons ejected from the metal in the photocell had a maximum kinetic energy of 4.9 eV.
Based on this information and Table 1, the relative intensity of the light shone on the photocell: