Section: Science
158)
Paper chromatography can be used to identify metal ions in wastewater. A drop of sample solution is placed on filter paper. The bottom of the paper is set in a solvent that travels up the paper (see Figure 1).
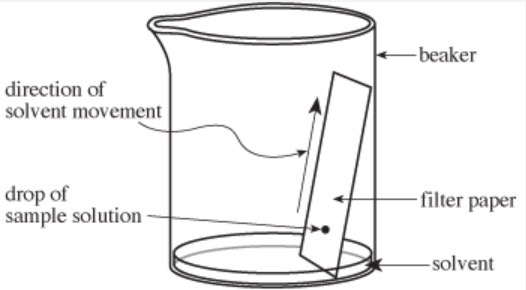
The solvent carries the ions up the paper. Some ions move faster, and therefore farther than others, resulting in a separation as they move up the paper. The paper is dried, then stained, causing the ions to appear as colored spots. Rf values are calculated for each spot:

Table 1 shows Rf values for 5 ions. Table 2 shows Rf values from 3 samples of wastewater. The same solvent was used for all ions and samples.
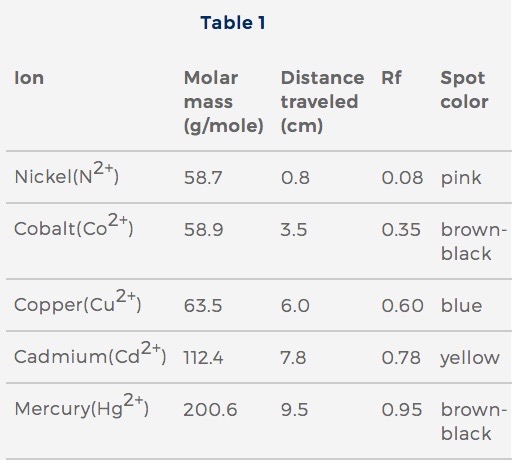
Table 1 adapted from Thomas McCullough, CSC, and Marissa Curlee, “Qualitative Analysis of Cations Using Paper Chromatography.” ©1993 by the American Chemical Society.
Note: Samples contain only the metal ions listed in Table 1.
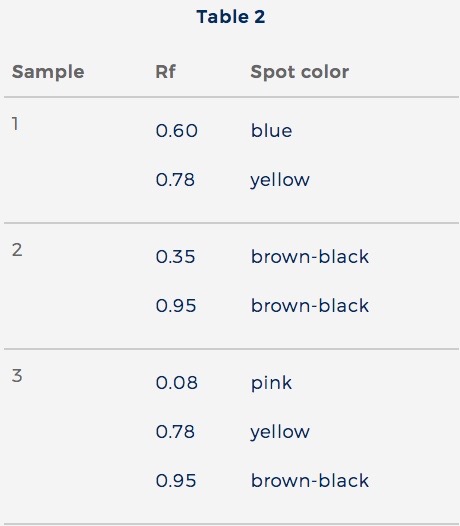
The information in Tables 1 and 2 supports the conclusion that Sample 3 contains:
-
-
-
-
Explanation
Answer 4 is correct. Sample 3 had 3 colored spots: a pink spot with an Rf = 0.08, a yellow spot with an Rf = 0.78, and a brown-black spot with an Rf = 0.95. These 3 spots correspond with 3 spots from Table 1: the pink spot with an Rf = 0.08 for Ni2+, the yellow spot with an Rf = 0.78 for Cd2+, and the brown-black spot with an Rf = 0.95 for Hg2+. So Sample 3 contains Ni2+, Cd2+, and Hg2+.